 Uncapped
mRNA introduced into tobacco protoplasts can be imported into the nucleus
and is trapped by leptomycin B Uncapped
mRNA introduced into tobacco protoplasts can be imported into the nucleus
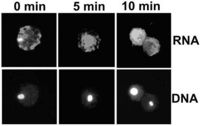
and is trapped by leptomycin BRogier Stuger and Christoph Forreiter Plant Cell Rep 23 (2004) 99-103 The mechanism of nuclear export of RNAs in yeast and animal cells is rapidly being uncovered, but RNA export in plants has received little attention. We introduced capped and uncapped fluorescent mRNAs into tobacco ( Nicotiana plumbaginifolia) protoplasts and studied their cellular localization. Following insertion, capped transcripts were found in the cytoplasm, while uncapped messengers transiently appeared in the nucleus in about one-quarter to one-third of the cells. These mRNAs were trapped by the nuclear export-inhibiting drug leptomycin B, pointing to an export mechanism in plants similar to Rev-NES-mediated RNP export in other organisms. [full-text pdf] |
 Messenger
RNA-binding properties of nonpolysomal ribonucleoproteins from heat-stressed
tomato cells
Messenger
RNA-binding properties of nonpolysomal ribonucleoproteins from heat-stressed
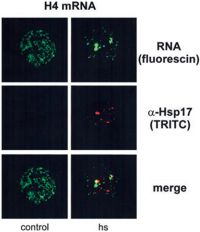
tomato cells